X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. With ARPES extension it is possible to measure also the emission angle and, consequently the k-vector information.
Laboratorio: nM2LAB
Running the X-ray source and electron analyzer requires high vacuum (P ~ 10−8 mbar) conditions in the analysis chamber and, therefore, conventional XPS is possible only under UHV conditions, making it difficult to conduct investigations of surfaces under real-world conditions (i.e. in the presence of gases and possibly liquids), such as is the case of interfacial chemical reactions in catalysis, chemical vapor deposition, electrochemistry and environmental chemistry. Near Ambient Pressure XPS (NAP-XPS) is an XPS system capable of operating at pressures of a few tens of mbar. We can now probe chemical interactions on the atomic level for vapor/solid interfaces. NAP-XPS also allows the investigation of the electronic and structural properties of small organics

TECHNICAL SPECIFICATIONS
XPS
- Monochromatized Al Kα, hν = 1486.6 eV, spot size 0.3 mm
- FWHM of Ag 3d = 0.8 eV (Epass = 20 eV)
- Recommended minimal atomic concentrations for analysis approx 5 % or higher
- Compensation of charging of samples in UHV (flood gun)
NAP cell
- Working pressures (N2 or equivalent): 0.05 – 5 mbar (optimal), pmax = 20 mbar, pmin = 1 x 10-4 mbar (indirect measurement of pressure, calibration needed)
Three gas inlets—two manuals (dosing of vapors of liquids and low pressures of
- gases) and one PC controlled with three mass flow controllers for premixing up to three gases (minimal pressure in NAP cell 0.5 mbar)
- Working temperatures: 200 – 850 K (in UHV, otherwise it depends on pressure and gas type, max. heating rate 0.2 K/sec, indirect conductive heating, no automatic control over temperature ramp, maximal temperature is limited to approx 750 K in oxygen and other oxidizing gases)
Preparation chamber (UHV manipulator)
- Working pressure: 10-9 mbar – 10-5 mbar
- Working temperatures: 100 – 1000 K (1300 K with specific sample holders, see below)
- Sample in vertical position, sample biasing, sample rotation
- UHV manipulator enable standard UHV XPS
SAMPLE
- Sample lateral dimensions: 10 x 5 mm (ideal), 3 x 3 mm (minimal), 10 x 10 mm (maximal)
- Sample thickness: ideally up to 2 mm (thicker samples also feasible)
- Sufficient electrical conductivity of samples needed to avoid charging (charging can be partially compensated by presence of gas atmosphere, especially at elevated temperatures)
- Mechanical integrity of samples needed because they are measured in vertical position (powder samples should be in a form of pellets or pressed into soft metal foil or mesh)
- No availability for liquid samples
- Time for loading the sample and its transfer to measuring position is 30 min
AVAILABLE TECHNIQUES
XPS
- XPS survey in the energy range xx-yy
- Chemical composition chemical bonding of the surface wit resolution of XXX
- ……
ARPES
- Valence band dispersion
XPS-NAP
- Analysis of samples in the presence of a gas (or mixture of gases) up to a total pressure of 25mbar. Currently available gases: CO2, H2O, O2, H2, CO, NH3. Other gases may be possible by request.
- Heating/cooling of samples from ~ 0ºC to 700ºC during analysis.
USE FOR
- Semiconductor/ Microelectronics
- Microcircuits
- Ultra-thin Films
- Soldering
- Cleaning
- Thin-film Stability
- Barrier Layers
- Lubrication
- Chemical Industry
- Plastics/Coatings
- Catalysis
CASE STUDIES
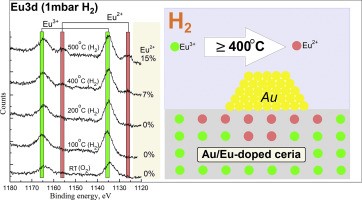
NAP-XPS study of Eu3+ → Eu2+ and Ce4+ → Ce3+ reduction in Au/Ce0.80Eu0.20O2 catalyst Eu3+ → Eu2+ and Ce4+ → Ce3+ reduction in Au/Ce0.80Eu0.20O2 catalyst were systematically studied using in-situ NAP-XPS technique. The reduction of Eu3+ → Eu2+ in the ceria, in a hydrogen atmosphere at temperature ≥ 400 °C, has been demonstrated for the first time. The effect of the reduction of Ce0.80Eu0.20O2 support on the catalytic activity of Au nanoparticles in CO oxidation has been investigated.
CASE STUDIES
Instability in CH3NH3PbI3 perovskite solar cells due to elemental migration and chemical composition changes
Organic-inorganic halide perovskites have rapidly grown as favorable materials for photovoltaic applications, but accomplishing long-term stability is still a major research problem. This work demonstrates a new insight on instability and degradation factors in CH3NH3PbI3 perovskite solar cells aging with time in open air. X-ray photoelectron spectroscopy (XPS) has been used to investigate the compositional changes caused by device degradation over the period of 1000 hrs.